Answer:
2NaHCO3(aq)+H2SO4(aq)→Na2SO4(aq)+2CO2(g)+2H2O(l)
Step-by-step explanation:
As we know that
acid + carbonate → salt + carbon dioxide + water
So, the general (un-balanced) equation would be-
NaHCO3(aq)+H2SO4(aq)→Na2SO4(aq)+CO2(g)+H2O(l)
Now we will write the net ionic reactions
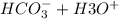 ----> CO2(g)↑+2H2O(l)
----> CO2(g)↑+2H2O(l)
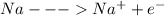
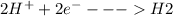

Adding all the above equation, we get
2NaHCO3(aq)+H2SO4(aq)→Na2SO4(aq)+2CO2(g)+2H2O(l)