Answer: Yes , in
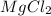 , two electrons from magnesium are transferred to chlorine atoms.
, two electrons from magnesium are transferred to chlorine atoms.
Step-by-step explanation:
Ionic compound is formed by the transfer of electrons. For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
For example : For formation of
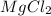
Electronic configuration of magnesium:
![[Mg]=1s^22s^22p^63s^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/iqkipx3qptj0cmx7efr7amh41fwulp0ddq.png)
Magnesium atom will loose two electrons to gain noble gas configuration and form magnesium cation with +2 charge.
![[Mg^(2+)]=1s^22s^22p^63s^0](https://img.qammunity.org/2021/formulas/chemistry/high-school/2umv2twztyrbmbk7104erykmkjrrgi1km0.png)
Electronic configuration of chlorine:
![[Cl]=1s^22s^22p^63s^23p^5](https://img.qammunity.org/2021/formulas/chemistry/high-school/5clg4okbri6qqmvq2nc4bxyw2knzg5mham.png)
Chlorine atom will gain one electron to gain noble gas configuration and form chloride ion with -1 charge.
![[Cl^-]=1s^22s^22p^63s^23p^6](https://img.qammunity.org/2021/formulas/chemistry/high-school/z89m1j5a61w68f9wbx2s9q5qrne1sppaeb.png)
In Magnesium chloride two electrons from magnesium metal gets transferred to two chlorine atoms and thus
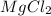 is formed.
is formed.