Answer:
Option C, (Actual yield ÷ percent yield) × 100
Step-by-step explanation:
Theoretical yield is defined as the total amount of product formed for given reactants in a chemical reaction. It is an ideal case which assumes no exceptions or wastage.
The mathematical relation between the actual yield, percent yield and theoretical yield is as follows -
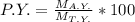
Where
P.Y. represents the percent yield a
M A.Y. represents the mass obtained from actual yield
M T.Y. represents the mass obtained from theoretical yield
Hence, if we rearrange the formula, we get -
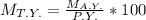
Hence, option C is correct