Answer:
The new volume of the balloon be
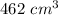 .
.
Step-by-step explanation:
We have,
Initial volume is 556 cm ³
The temperature of the balloon decreases from 278 K to 231 K.
We need to find the new volume of the balloon if the pressure is constant.
The Charles law states that at constant pressure, the volume of gas is directly proportional to its temperature i.e.
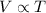 , P is constant
, P is constant
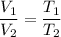
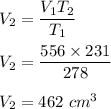
So, the new volume of the balloon be
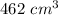 .
.