Answer:
About 0.1738 liters
Step-by-step explanation:
Using the formula PV=nRT, where p represents pressure in atmospheres, v represents volume in liters, n represents the number of moles of ideal gas, R represents the ideal gas constant, and T represents the temperature in kelvin, you can solve this problem. But first, you need to convert to the proper units. 215ml=0.215L, 86.4kPa is about 0.8527 atmospheres, and 15C is 288K. Plugging this into the equation, you get:
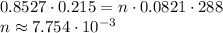
Now that you know the number of moles of gas, you can plug back into the equation with STP conditions:
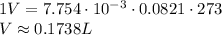
Hope this helps!