Answer:
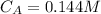
Step-by-step explanation:
Hello,
In this case, given the rate constant, we infer this is about a second order reaction, whose equation is:
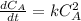
Thus, by integration we obtain:
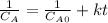
Hence, we solve for the final concentration after 0.385 s:
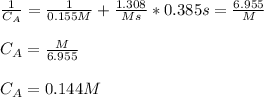
Best regards.