Answer:
It is an example of double displacement reaction.
4.8 g of NaCl is needed to react.
Step-by-step explanation:
Balanced reaction:
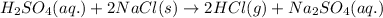
Here, oxidation states of H, S, O, Na and Cl do not change during reaction. Hence it is not a redox reaction.
In this reaction, cations and anions of the reactants interchange their partners during reaction. Hence, it is an example of double displacement reaction.
As
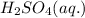 remain in excess amount therefore NaCl (s) is the limiting reagent. Hence production of HCl entirely depends on amount of NaCl used.
remain in excess amount therefore NaCl (s) is the limiting reagent. Hence production of HCl entirely depends on amount of NaCl used.
Molar mass of HCl = 36.46 g/mol
So, 3.0 g of HCl =
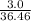 mol of HCl = 0.082 mol of HCl
mol of HCl = 0.082 mol of HCl
According to balanced equation-
2 moles of HCl are produced from 2 moles of NaCl
So, 0.082 moles of HCl are produced from 0.082 moles of NaCl
Molar mass of NaCl = 58.44 g/mol
So, mass of 0.082 moles of NaCl =
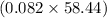 g = 4.8 g
g = 4.8 g
Hence 4.8 g of NaCl is needed to react.