Answer:
The amount of mass that needs to be converted to release that amount of energy is
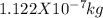
Step-by-step explanation:
From Albert Einstein's Energy equation, we can understand that mass can get converted to energy, using the formula

where
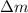 = change in mass
= change in mass
c = speed of light =
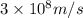
Making m the subject of the formula, we can find the change in mass to be
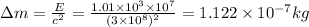
There fore, the amount of mass that needs to be converted to release that amount of energy is 1.122 X 10 ^-7 kg