Answer:
Mass, m = 105.58 g
Step-by-step explanation:
We have,
Heat required in aluminium to change the temperature from 68°C to 110°C. It is required to find the mass of aluminium.
Concept used : Specific heat capacity
Solution,
The heat required to raise the temperature is given by :
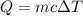
c is specific heat capacity, for Aluminium, c = 0.902 J/g-°C
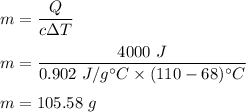
So, the mass of aluminium is 105.58 grams.