Answer:
Step-by-step explanation:
Part A
- Work function ( ∅ ) is the minimum energy required by the photon to knock an electron out of the metal surface. That is the portion of energy of a photon transferred to an electron so that it can escape the metal.
- We can mathematically express it:
∅ = Ep - Ek
=
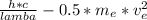
Where,
Planck's constant ( h ) = 6.6261*10^-34
Speed of light ( c ) = 3*10^8 m/s
mass of an electron ( m_e ) = 9.1094*10^-31 kg
Given:-
Incident light's wavelength ( λ ) = 250*10^-9 m
The maximum speed o electron ( v_e ) = 4*10^5 m/s
Solution:-
- Plug the values into the expression derived before:
∅ =
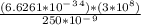
∅ =
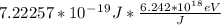
∅ = 4.508 eV ... Answer
Part B
- 2.5% of the energy emitted by a 100-W light bulb was visible light with wavelength ( λ ) = 500*10^-9 m. In 1.0 min the amount of energy harbored by a stream of photons are:
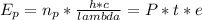
Where,
Planck's constant ( h ) = 6.6261*10^-34
Speed of light ( c ) = 3*10^8 m/s
Given:-
visible light's wavelength ( λ ) = 500*10^-9 m
Power of light bulb ( P ) = 100 W
Time taken ( t ) = 1.0 min = 60 s
Portion of energy as light ( e ) = 0.025
Solution:-
- Plug the values into the expression derived before:
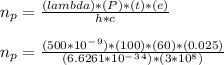
n_p = 3.773 * 10^20 ... Answer
Part C
- A blood vessel of radius ( r ) with length ( L ) carries blood with viscosity ( μ ). The pressure drop ( ΔP ) in the blood vessel was witnessed.
- Pressure loss ( ΔP ) in a cylindrical blood vessel is given by the Darcy's equation given below:
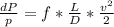
Where,
ρ: Density of blood
f: Friction factor
D: Diameter of vessel
v: Average velocity
- The friction factor is a function of Reynolds number and relative roughness of blood vessel. We will assume the blood vessel to be smooth, round and the flow to be laminar ( later verified ).
- The flow rate ( Q ) in a smooth blood vessel subjected to laminar flow conditions is given by the Poiseuille's Law. The law states:
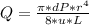
- The velocity ( v ) in a circular tube is given by the following relation:
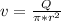
Given:-
dP ( Pressure loss ) = 2.5 Pa
radius of vessel ( r ) = 10μm = 10*10^-6 m
viscosity of blood ( μ ) = 0.0027 Pa.s
Length of vessel ( L ) = 1μm = 10^-6 m
Solution:-
- Use the Poiseuille's Law to determine the flow rate ( Q ) of the blood in the vessel:
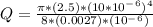
Q = 3.6361*10^-12 m^3 / s
- The corresponding velocity ( v ) of the blood flow would be:
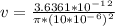
v = 0.01157 m/s
- Use the Poiseuille's Law to determine the flow rate ( Q ) of the blood blood in the enlarged vessel ( r = 12 μm = 10*10^-6 m ) :
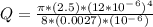
Q = 7.54*10^-12 m^3 / s
- The corresponding velocity ( v ) of the blood flow would be:
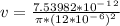
v = 0.01666 m/s
Part D
- A radioactive isotope of Cobalt ( Co - 60 ) undegoes Beta decay ( 0.31 MeV ) and emits two gamma rays of energy ( 1.17 & 1.33 ) MeV.
- The radioactive decay for the ( Cobalt - 60 ) can be expressed in form of an equation:
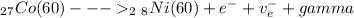
- The half life ( T_1/2 ) of the Co-60 can be used to determine the decay constant ( λ ):
λ =
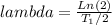
Where,
T_1/2 = 5.2 yrs = 1.68*10^8 s
Hence, the decay constant is
λ =
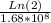 = 4.22*10^-9 s^-1
= 4.22*10^-9 s^-1
- The activity ( A ) of any radioactive isotope is function of time ( t ) defined by negative exponential distribution:
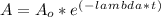
Where,
A_o: The initial activity ( Bq )
- The activity of the radioactive isotope Co-60 was A = 10 Ci after t = 30 months. The initial activity ( A_o ) can be determined:
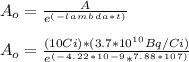
A_o = 5.016 * 10^11 Bq
- The initial number of nuclei in the sample ( N_o ) is given by:
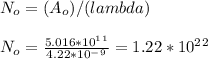
- The initial mass of Co-60 used as a sample can be determined:
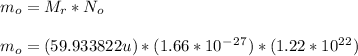
m_o = 12.2 * 10^-6 kg ... Answer
- The total energy ( E ) released from the beta decay transformation:
E = E(β) + E(γ1) + E(γ2) = 0.31 + 1.17 + 1.33 = 2.81 MeV
- The rate at which the source emits energy after 30 months:
P = E*A =
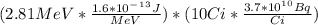
P = 0.166 W .. Answer