Answer:
The number of atoms in 10 g of Pb is 2.9064 × 10²² atoms
Step-by-step explanation:
According to Avogadro's constant, one mole of a substance contains exactly 6.02214076 × 10²³ particles such as molecules, atoms or ions.
Here we have lead, Pb, which is an uncombined element, therefore, the number of moles present in 10 g of lead can be found as follows;
Molar mass of lead = 207.2 g/mol
Mass of lead sample = 10 g
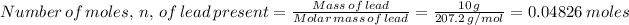
Therefore, number of atoms in 10 g of lead is found by multiplying the number of moles by the Avogadro's number as follows;
Number of atoms = Number of moles × Avogadro's number
∴ Number of atoms in 10 g of Pb = 0.04826 × 6.02214076 × 10²³ = 2.9064 × 10²² atoms.
The number of atoms in 10 g of Pb = 2.9064 × 10²² atoms.