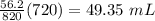Answer:
49.35 mL
Step-by-step explanation:
Given: 56.2 mL of gas
To find: volume that 56.2 mL of gas at 820 mm of Hg would occupy at 720 mm of Hg
Solution:
At 820 mm of Hg, volume of gas is 56.2 mL
At 1 mm of Hg, volume of gas is
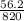
At 720 mm of Hg, volume of gas is