Answer:
The energy a 15,000 g block of ice will absorb in order to melt is 4995 kJ
Explanation:
The question relates to the latent heat of fusion of ice, that is the heat absorbed by the solid ice to convert it to liquid water without changing its temperature.
The formula for the energy required to melt the ice is presented as follows;
Energy required = Mass, m × Latent heat of fusion,
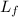 , of ice
, of ice
Hence, the mass, m, of the ice = 15,000 g
Latent heat of fusion,
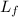 , of ice = 333 J/g
, of ice = 333 J/g
Therefore;
Energy required = 15,000 g × 333 J/g = 4995000 J
Hence;
The energy a 15,000 g block of ice will absorb in order to melt = 4995000 J
1000 J = 1 kJ
1 J = 1/1000 kJ
Therefore, 4995000 J = (4995000 J)/(1000 J/kJ) = 4995 kJ.
Which gives;
The energy a 15,000 g block of ice will absorb in order to melt = 4995 kJ.