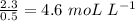Answer:
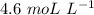
Step-by-step explanation:
Molarity refers to a measure of concentration.
Molarity = moles of solute/Litres of solution
Molarity refers to number of moles of solute present in this solution.
In order to find a solution's molarity, use value for the number of moles of solute and the total volume of the solution expressed in liters
As molarity of 2.3 mol of Kl is dissolved in 0.5 L of water,
Molarity =