Answer:
The amount in grams of the excess reactant (NO) used in the chemical reaction is 30 grams
Step-by-step explanation:
The balanced chemical equation for the reaction is presented as follows;
O₂ (g) + 2NO (g) → 2NO₂ (g)
Therefore, one mole of O₂ combines with two moles of NO to produce two moles NO₂
Molar mass of oxygen gas, O₂ = 31.999 g/mol
Mass of oxygen gas, O₂, present = 16.00 g
Molar mass of nitric oxide, NO = 30.01 g/mol
Mass of nitric oxide, NO, present = 80.008
Number of moles, n, of a sample of a substance is given by the following relation;
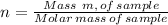
Number of moles of O₂ present is presented as follows;
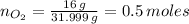
Number of moles of NO present is presented as follows;
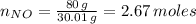
Hence, since one mole of O₂ combines with two moles of NO to produce two moles NO₂, 0.5 moles of O₂ will combine with one mole of NO to produce one mole NO₂. Therefore, the oxygen is used up in the reaction and the nitric oxide is the excess reactant.
Number of moles of nitric oxide, NO, consumed in the reaction = 1 mole
Mass, m of nitric oxide used = number of moles of nitric oxide × molar mass of nitric oxide
∴ Mass of nitric oxide used = 1 mole × 30.01 g/mol = 30.01 grams
Rounding to the nearest whole number, we have;
Mass of nitric oxide used = 30 grams.
The amount in grams of the excess reactant (NO) used in the chemical reaction = 30 grams.