Answer:
9.28
Step-by-step explanation:
pOH refers to a measure of hydroxide ions concentration. pOH tells about the alkalinity of a solution. If pOH is less than 7 then aqueous solutions are alkaline, acidic if pOH is greater than 7 and neutral if pOH is equal to 7.
Concentration of the hydroxide ions = 1.9 x 10-5 M
pH =
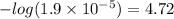
pOH = 14 - pH
=14 - 4.72 = 9.28