Answer:
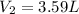
Step-by-step explanation:
Hello,
In this case, we can use the combined ideal gas law avoiding the usage of the universal ideal gas constant in order to understand the volume-pressure-temperature behavior at constant moles as given on the statement:
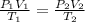
Thus, since the temperature must be operated in absolute Kelvin units, we compute the required volume at 1.54 atm and 10.0 °C, considering that the initial STP conditions are 1 atm and 0 °C:
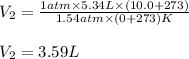
Best regards.