Answer:
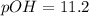
Step-by-step explanation:
Hello,
In this case, by knowing that the pOH is defined in terms of the concentration of OH⁻ as shown below:
![pOH=-log([OH^-])](https://img.qammunity.org/2021/formulas/chemistry/college/os6i8jhsq2yoevim3ewlj1jvyoxoba6pi1.png)
We directly compute with the given concentration:
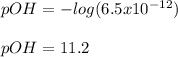
Moreover, fur such pOH, the pH will be:
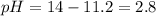
Which means that such solution is an acid solution.
Best regards.