Answer:
The new volume is 2249.28 mL.
Step-by-step explanation:
According to Avogadro's Law, the number of moles in a gas is directly proportional to the volume.
Initial no of moles are 0.504 moles at a volume of 2680 mL.
It is required to find the volume if number of moles changes to 0.423 moles.
Using Avogadro's Law,
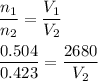
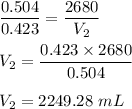
So, the new volume is 2249.28 mL.