Answer:
1)
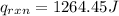
2)
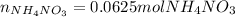
3)
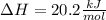
Step-by-step explanation:
Hello,
In this case, with the given data, we proceed as shown below:
1) q for the reaction is computed by considering the total mass of the system, that is the mass of ammonium nitrate and water:
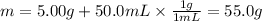
Next, by using the heat capacity of the solution and the change in temperature, we obtain heat of solution:
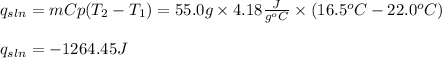
Finally, the heat of reaction:
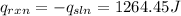
2) For the moles of solid ammonium nitrate we use its molar mass (80.05 g/mol) and the used 5.00 g:
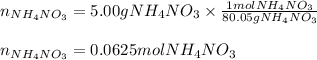
3) Finally, we obtain the change in the enthalpy of reaction by using heat of reaction and the reacted moles of ammonium nitrate:
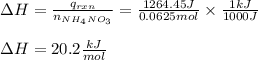
Best regards.