Answer:
pH of the gastric juice
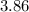
Step-by-step explanation:
As we know,
In an acid base titration, the number of milimoles of acid is equal to the number of milimoles of base.
Milimoles
 Molarity
Molarity
 Volume
Volume
Molarity of gastric juices
 Volume of gastric juices
Volume of gastric juices
 Molarity of KOH
Molarity of KOH
 Volume of KOH
Volume of KOH
Substituting the given values in above equation, we get -
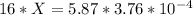
X represents the molarity of gastric juices
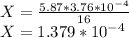
pH of gastric juices is equal to log

![= - log 1.379 * 10^(-4)\\= - [-3.86]\\= 3.86](https://img.qammunity.org/2021/formulas/biology/college/88heyw409ecdsrs2surjd0s18qluh1a0td.png)