Answer:
16.67 grams of H₂ is generated from the electrolysis of 150 grams of H₂O
Step-by-step explanation:
Electrolysis is the decomposition of a chemical element under the effect of an electric current. So, electrolysis of water is the process of decomposing the H₂O molecule into separate oxygen and hydrogen gases due to an electric current passing through the water.
The balanced equation of electrolysis of water is:
2 H₂O → O₂ + 2H₂
Being:
then the molar mass of the compounds that participate in the reaction is:
- H₂O: 2*1 g/mole + 16 g/mole= 18 g/mole
- H₂: 2*1 g/mole= 2 g/mole
- O₂: 2*16 g/mole= 32 g/mole
If the following amounts in moles are reacted by stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction):
- H₂O: 2 moles
- H₂: 2 moles
- O₂: 1 mole
the amount of mass, by stoichiometry, that reacts and is produced is:
- H₂O: 2 moles*18 g/mole=36 g
- H₂: 2 moles* 2 g/mole= 4 g
- O₂: 1 mole* 32 g/mole= 32 g
Then you can apply the following rule of three: if by stoichiometry 36 g of H₂O generate 4 g of H₂, 150 g of H₂O how much mass of H₂ will it generate?
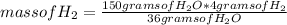
mass of H₂= 16.67 grams
16.67 grams of H₂ is generated from the electrolysis of 150 grams of H₂O