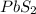Answer: The chemical formula for the compound of these two elements is
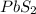
Step-by-step explanation:
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
Here metal lead is having an oxidation state of +4 called as
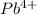 cation and sulphur non metal has oxidation state of -2 called as
cation and sulphur non metal has oxidation state of -2 called as
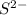 . Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
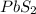
The chemical formula for the compound of these two elements is