Answer:
A
Step-by-step explanation:
HCl and NaOH is a strong acid and strong base, respectively, and hence dissociates completely and reacts to form water:
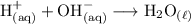
We are given that 25.0 mL of 0.333 M NaOH was used to neutralize 15.0 mL of HCl.
Convert from moles of NaOH used to moles of HCl reacted:
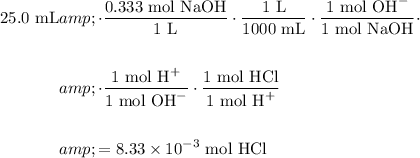
Therefore, the molarity of the original HCl solution is:
![\displaystyle \begin{aligned} \ [\text{HCl}] & = \frac{\text{ mol HCl}}{\text{L soln.}} \\ \\ & =\frac{8.33* 10^(-3)\text{ mol HCl}}{15.0\text{ mL}} \cdot \frac{1000\text{ mL}}{1\text{ L}} \\ \\ & = 0.555\text{ M}\end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/high-school/ztqmvnztl1iorhll8y1rvaqf11ew92o3im.png)
In conclusion, our answer is A.