Answer:
in the presence of a base
Step-by-step explanation:
Phenolphthalein will turn pink in the presence of a base.
Phenolphthalein is an indicator that is used in acid/base titration to indicate end points. It responds to pH changes by changing its color. In basic solutions, the compound turns pink while it remains colorless in acidic medium. It has the chemical formula
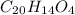 .
.
Phenolphthalein is considered a weak acid ordinarily and it ionizes incompletely in aqueous solution.
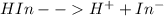
Addition of hydrogen ions shifts equilibrium to the left and the solution remains colorless while the addition of hydroxide ion removes the hydrogen ion and shifts the equilibrium to the right, giving pink coloration.