Answer:
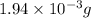 of NaOH
of NaOH
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rjo2yhb5oj9ry1fr4db1ujrazm6fh3vhke.png)
Putting in the values:
![10.020=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/d6ob0wct0qqcy62vo1mhhutgejv2cj5guh.png)
![[H^+]=9.55* 10^(-11)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/a31euq6ahsnz67qvfusd1v4lptpctcqolp.png)
![[H^+][OH^-]=10^(-14)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/qlgpp64i9ug80dgrifxhx8udhf64qlr76a.png)
![[OH^-]=(10^(-14))/(9.55* 10^(-11))=1.05* 10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/middle-school/5yxwzyx8z5zms5rwv9rtit1z7d6xbq0x5r.png)
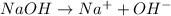
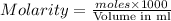
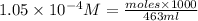
moles =
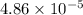
Mass of
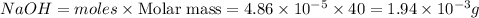
Thus
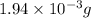 of NaOH is needed to prepare 463 mL of solution with a pH of 10.020
of NaOH is needed to prepare 463 mL of solution with a pH of 10.020