Answer:
![[H_3O^+]=2.40x10^(-13)M](https://img.qammunity.org/2021/formulas/chemistry/college/ox68nas4qsmik8h04ijo3gzhoi1vu54jh2.png)
![[OH^-]=0.0417M](https://img.qammunity.org/2021/formulas/chemistry/college/puv6dn1kwmq76dhkxdgm4hdgby27dvpzk7.png)
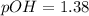
Step-by-step explanation:
Hello,
In this case, we are able to compute the concentration of hydroxyl and hydronium by using both he pH and the pOH as shown below:
![[OH^-]=10^(-pOH)](https://img.qammunity.org/2021/formulas/chemistry/college/o2ob2jyphyjxp6ykyoywmx5rl9j3ypyvyd.png)
![[H_3O^+]=10^(-pH)](https://img.qammunity.org/2021/formulas/chemistry/college/kk3407eh2gvrkie4vbpyjiei5hmefyfv8e.png)
Moreover, we are able to compute the pOH by:
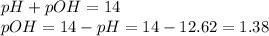
So we compute the concentrations:
![[OH^-]=10^(-1.38)=0.0417M](https://img.qammunity.org/2021/formulas/chemistry/college/jdit81u77awrn7cljp0m5mhscg5ou9b5c1.png)
![[H_3O^+]=10^(-12.62)=2.40x10^(-13)M](https://img.qammunity.org/2021/formulas/chemistry/college/rwtqr0aahgn1b48d2wk37plt26h6zwqbi9.png)
Best regards.