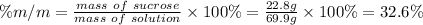Answer:
32.6 %
Step-by-step explanation:
Given data
- Mass of sucrose (solute): 22.8 grams
- Mass of water (solvent): 47.1 grams
Step 1: Calculate the mass of the solution
The mass of the solution is equal to the sum of the mass of the solute and the mass of the solvent.
m(solution) = m(solute) + m(solvent)
m(solution) = 22.8 g + 47.1 g
m(solution) = 69.9 g
Step 2: Calculate the percent-by-mass of sucrose in the solution
We will use the following expression.