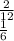Answer:
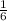
Step-by-step explanation:
Molar ratio is defined as the ratio of number of moles of reactants or products in a balanced chemical equation.
In this balanced equation, the number of C6H6 molecules react to form 12 CO2 molecules.
Thus, the molar ratio between C6H6 to carbon will be equal to 2 moles of C6H6 divided by 12 moles of CO2