Answer:
The rank of temperature from lowest to highest is
Lowest --- Middle --- Highest
Water --- Glass --- Iron
Step-by-step explanation:
From the question we are told that
The mass of iron, glass, and water is
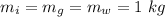
The temperature of iron, glass, and water is
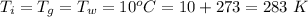
The energy added is
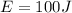
The specific heat of iron is a constant with a value
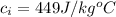
The specific heat of glass is a constant with a value
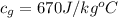
The specific heat of water is a constant with a value
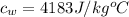
Now energy transferred to these substances can be mathematically represented as
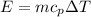
Now from the question the mass of the substance and the energy added are constant
So
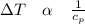
Thi means that the change in temperature is inversely proportional to the specific heat of the substance
With this knowledge we can deduce that
The substance with the highest temperature is Iron
followed by glass
and then water