Answer:
pH=10.88
Step-by-step explanation:
Hello,
In this case, since potassium hydroxide is completely dissociated as shown below:
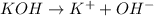
For which we understand it is a base, more specifically, a strong base; it means that the concentration of the OH⁻ equals the concentration of the potassium hydroxide, that is 0.000765M, for that reason we can directly compute the pOH:
![pOH=-log([OH^-])=-log(0.000765)=3.12](https://img.qammunity.org/2021/formulas/chemistry/college/sq9xa84p3ayjxxxu21oaz5rregb7ddycz8.png)
Finally, since the pOH and the pH are related by:
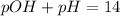
The pH turns out:
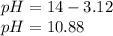
Best regards.