Answer:
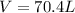
Step-by-step explanation:
Hello,
In this case, butane's combustion is represented by the following chemical reaction:
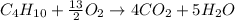
Which is typically carried out in gas phase. Thus, for 45.6 g of butane, we employ its 1:4 molar relationship in the chemical reaction to compute the yielded moles of carbon dioxide by stoichiometry factors:
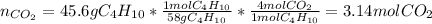
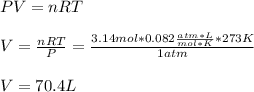
Next, by using the ideal gas equation at STP (273 K and 1 atm), we compute the produced liters of carbon dioxide as shown below:
Best regards.