Answer:
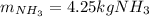
Step-by-step explanation:
Hello,
In this case, for the production of ammonium nitrate we shall consider the following chemical reaction:
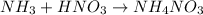
Hence, since the molar mass of ammonium nitrate is 80 g/mol and the molar mass of ammonia is 17 g/mol, we could compute the required mass of ammonia to produce 20 kg of ammonium nitrate by using kilo-based units:
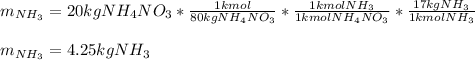
Best regards.