Answer:
Oxygen O₂ will be the limiting reagent.
Step-by-step explanation:
C₂H₆O(g) + 3 O₂(g) ⇒ 2 CO₂(g) + 3 H₂O(g)
First of all you must know by stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction) how much mass of each compound reacts. First of all, being:
- C: 12 g/mole
- H: 1 g/mole
- O: 16 g/mole
the molar mass of each reagent is:
- C₂H₆O: 2*12 g/mole + 6* 1 g/mole + 16 g/mole= 46 g/mole
- O₂: 2*16 g/mole= 32 g/mole
Then, since 1 mol of C₂H₆O and 3 moles of O₂ react by stoichiometry, the amount of mass that reacts is:
- C₂H₆O: 1 mole* 46 g/mole= 46 g
- O₂: 3* 32 g/mole= 96 g
Now you apply a rule of three as follows: if 46 g of C₂H₆O reacts with 96 g of O₂ by stoichiometry, 0.461 g of C₂H₆O with how much mass of O₂ will they react?
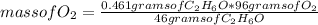
mass of O₂= 0.962 grams
But 0.962 grams of O₂ are not available, 0.640 grams are available. Since you have less mass than you need to react with 0.461 g of C₂H₆O, oxygen O₂ will be the limiting reagent.