Answer:
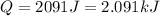
Step-by-step explanation:
Hello,
In this case, the formula we use to compute the heat Q by increasing the temperature, in terms of the mass and the heat capacity is:
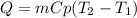
Titanium's heat capacity is 0.544284 J/g°C, thus, the for such temperature increase, the heat results positive as shown below:
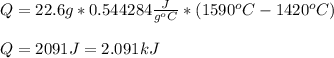
Best regards.