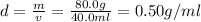Answer:
The correct answer is (B) 0.50 g/mL
Step-by-step explanation:
The density of a subtance is equal to the ratio between its mass and its volume, as follows:
d = m/v
In the problem, the mass is equal to 80.0 g. The volume is given by the increment in volume in the graduated cylinder when the solid is placed because the solid is insoluble so it displaces the liquid and increases the volume. The initial volume was 40.0 ml and the final volume was 80.0 ml, so:
V = Final volume - initial volume = 80.0 ml - 40.0 ml = 40.0 ml
Finally, we calculate the density by dividing the mass into the volume: