Answer:
1/2 of the Pi
Step-by-step explanation:
PV = nRT
we are going to assume the only things changing are pressure and number of mols, so we change the formula and substitute the variables to make it what we are looking for
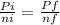
then we will assume Pi as 1 as it is not given, so we can state it easier
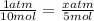
then either cross multiply or just work it out to be
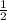 which we can say as "half of the initial pressure" as no number is given for initial pressure
which we can say as "half of the initial pressure" as no number is given for initial pressure