Answer:
A) 12 mol H2O.
Step-by-step explanation:
Hello,
In this case, for the given reaction:
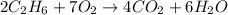
We notice that oxygen is in a 7:6 molar relationship with water, for that reason, the resulting moles of water turn out:
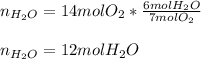
Thus, the answer is A) 12 mol H2O.
Best regards.