Answer:
The limiting reactant is the propane gas, C₃H₈ while the percentage yield is 83.77%
Step-by-step explanation:
Here we have
Propane gas with molecular formula C₃H₈, molar mass = 44.1 g/mol combining with O₂ as follows
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Therefore, 1 mole of C₃H₈ combines with 5 moles of O₂ to produce 3 moles CO₂ and 4 moles of H₂O
Mass of propane = 0.1240 kg = 124.0 g
Number of moles of propane = mass of propane/(molar mass of propane)
The number of moles of propane = 124/44.1 = 2.812 moles
The molar mass of CO₂ = 44.01 g/mol
Mass of CO₂ = 0.3110 kg = 311.0 g
Therefore, number of moles of CO₂ = mass of CO₂/(molar mass of CO₂)
The number of moles of CO₂ = 311.0 kg/ 44.01 g/mol = 7.067 moles
Therefore, since 1 mole of propane produces 3 moles of CO₂, 2.812 moles of propane will produce 3 × 2.812 moles or 8.44 moles of CO₂
Therefore;
The limiting reactant is the propane gas, C₃H₈, since the oxygen is in excess
Hence
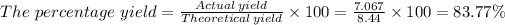
The percentage yield = 83.77%.