Answer:
If you have 67.31 g of CH₄, you have 4.21 moles
Step-by-step explanation:
To know the amount of moles if you have 67.31 g of CH₄, you must know the molar mass, that is, the mass of one mole of a substance, which can be an element or a compound.
On the periodic table, the molar mass of the elements, also called the atomic mass or atomic weight, can be found at the bottom of the element. In this case:
To calculate the molar mass of a compound, the molar mass of the elements of the compound must be added multiplied by the times they appear. So in this case the molar mass of CH₄ is:
CH₄= 12 g/mole + 4* 1 g/mole= 16 g/mole
Now you can apply the following rule of three: if 16 g are contained in 1 mole of CH4, 67.31 g in how many moles are present?
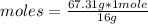
moles= 4.21
If you have 67.31 g of CH₄, you have 4.21 moles