Answer:
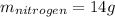
Step-by-step explanation:
Hello,
In this case, since we are talking about a chemical reaction in which a compound having nitrogen and chlorine is decomposed into chlorine and nitrogen, we must remember that the law of conservation of mass must be obeyed, for that reason, we notice that the mass of the whole reactants must equal the mass of the whole products, as shown below:
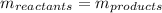
Next, we know there is only one single reactant and products are constituted by both chlorine and nitrogen:
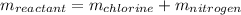
In such a way, we can compute the mass of nitrogen as shown below:
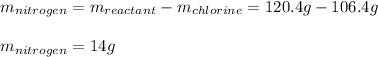
Best regards.