Answer:
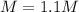
Step-by-step explanation:
Hello,
In this case, since we are mixing two NaOH solutions, the first step is to compute the total moles once the mixing is done, by using the volumes and concentrations of each solutions and subsequently adding them:
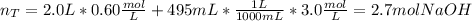
Next, we compute the total volume by adding the volume of each solution:
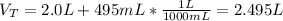
Finally, we compute the molarity of the resulting solution by the division between the total moles and the total volume:
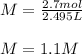
Best regards.