Answer:
The correct answer to the following question will be "0.0013%".
Step-by-step explanation:
The given values are:
Weight of an empty dish = 1.0041 ± 0.0002 g
Weight of dish + sodium chloride (NaCl) = 3.2933 ± 0.0002 g
Weight of NaCl = 2.2892 ± 0.0002 g
Now,
Volume of the solution = 500.00 ± 0.05 ± 0.0002
= 500.00 ± 0.0502 ml
So,
Molarity =
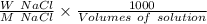
On putting the values in the above formula, we get
=
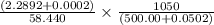
= (
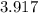 ±
±
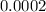 ) × (
) × (
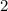 ±
±
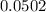 )
)
= (
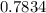 ±
±
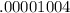 )
)
Now,
Absolute error =
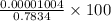
=
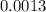 %
%