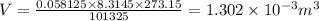Answer:
The volume occupied by the gas is 1.302 × 10⁻³ m³
Step-by-step explanation:
2HNO₃(aq) + Mg(s) → Mg(NO₃)₂ + H₂(g)
From the above reaction, we have that 2 moles of HNO₃ combine with 1 mole of Mg to produce 1 mole of Mg(NO₃)₂ and 1 mole of H₂
In the question, we have 155.0 mL of a solution of 0.75 M HNO₃ combining with 270 g of magnesium
Therefore, number of moles of HNO₃ = 0.75×155.0/1000 = 0.11625 moles
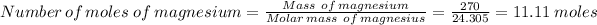
Therefore, HNO₃ is the limiting reaction, hence;
0.11625 moles of HNO₃ will react with 1/2×0.11625 moles or 0.058125 moles of Mg to produce 0.058125 moles of H₂
Hence since we now know the volume of H₂ produce, we can find the volume occupied at STP by the following universal gas equation relationship;
PV = nRT
Therefore;
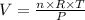
Where:
V = Volume of occupied by the gas = Required quantity
n = Number of moles = 0.058125 moles
R = Universal gas constant = 8.3145 J/(mol·K)
T = Temperature of the gas at STP = 273.15 K
P = Pressure of the gas at STP = 1 atm = 101325 Pa