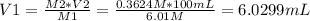Answer:
Volume of stock solution needed = 6.0299 mL
Step-by-step explanation:
Dilution consists of lowering the amount of solute per unit volume of solution. It is achieved by adding more diluent to the same amount of solute.
This is deduced when thinking that both the dissolution at the beginning and at the end will have the same amount of moles.
Data:
M1 = 6.01 M stock solution concentration
M2 = 0.3624 M diluted solution concentration
V2 =100 mL diluted solution volume
V1 = ? stock solution volume
M1 * V1 = M2 * V2