Answer:
The pressure is 4.8052 atmospheres.
Step-by-step explanation:
An ideal gas is a theoretical gas that is considered to be composed of point particles that move randomly and do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The state of a quantity of gaseous matter is formulated based on four different variables: pressure, volume, temperature and number of moles of gas. The equation known as the ideal gas equation explains the relationship between these four variables as follows:
P.V = n.R.T
where P represents the pressure of the gas, V its volume, n the number of moles of gas (which must remain constant), R the constant of the gases and T the temperature of the gas in question.
In this case:
- R= 0.082
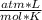
- T= 20 °C= 293 °K (being 0°C=273°K)
Replacing:
P* 2.50L=0.5 mol*0.082
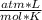 *293 °K
*293 °K
Solving:
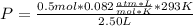
P=4.8052 atm
The pressure is 4.8052 atmospheres.