Answer:
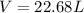
Step-by-step explanation:
Hello,
In this case, we use the ideal gas equation to compute the volume as shown below:
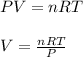
Nonetheless we are given mass, for that reason we must compute the moles of gaseous fluorine (molar mass: 38 g/mol) as shown below:
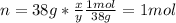
Thus, we compute the volume with the proper ideal gas constant, R:

Best regards.