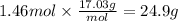Answer:
24.9 g
Step-by-step explanation:
Given data
- Volume of the solution: 1.75 L
- Concentration of ammonia: 0.833 M
Step 1: Calculate the moles of ammonia
The concentration of ammonia is 0.833 M, that is, there are 0.833 moles of ammonia (solute) per liter of solution. Then,
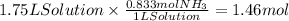
Step 2: Calculate the mass of ammonia
The molar mass of ammonia is 17.03 g/mol. The mass corresponding to 1.46 moles of ammonia is: