Answer:
1.155 moles of potassium nitrate are required to make 550 mL of a 2.1M solution.
Step-by-step explanation:
In a mixture, the chemical present in the greatest amount is called a solvent, while the other components are called solutes.
Molarity is a unit of concentration of a solution and indicates the amount of moles of solute that appear dissolved in each liter of the mixture. In other words, the Molarity (M) or Molar Concentration is the number of moles of solute that are dissolved in a given volume.
The Molarity of a solution is determined by the following expression:
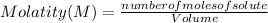
Molarity is expressed in units (
 ).
).
In this case:
- Molarity= 2.1 M
- number of moles of solute= ?
- Volume= 550 mL= 0.550 L (being 1L=1000 mL)
Replacing:
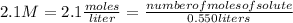
Solving:
number of moles of solute= 2.1 M* 0.550 L
number of moles of solute= 1.155 moles
1.155 moles of potassium nitrate are required to make 550 mL of a 2.1M solution.