Answer: 318 K
Step-by-step explanation:
Combined gas law is the combination of Boyle's law, Charles's law and Gay-Lussac's law.
The combined gas equation is,
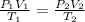
where,
 = initial pressure of gas = 231 kPa
= initial pressure of gas = 231 kPa
 = final pressure of gas = 168 kPa
= final pressure of gas = 168 kPa
 = initial volume of gas = 3.25 L
= initial volume of gas = 3.25 L
 = final volume of gas = 4.35 L
= final volume of gas = 4.35 L
 = initial temperature of gas =
= initial temperature of gas =
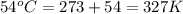
 = final temperature of gas = ?
= final temperature of gas = ?
Now put all the given values in the above equation, we get:
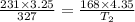

At 318 K of temperature will the same gas take up 4.35 liters of space and have a pressure of 168 kPa